The FDA Approval Process: A Gateway to Safety and Efficacy
Related Articles: The FDA Approval Process: A Gateway to Safety and Efficacy
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The FDA Approval Process: A Gateway to Safety and Efficacy. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: The FDA Approval Process: A Gateway to Safety and Efficacy
- 2 Introduction
- 3 The FDA Approval Process: A Gateway to Safety and Efficacy
- 3.1 Understanding the FDA Approval Process
- 3.2 The Importance of FDA Approval
- 3.3 Benefits of FDA-Approved Products
- 3.4 FAQs About FDA Approval
- 3.5 Tips for Consumers
- 3.6 Conclusion
- 4 Closure
The FDA Approval Process: A Gateway to Safety and Efficacy
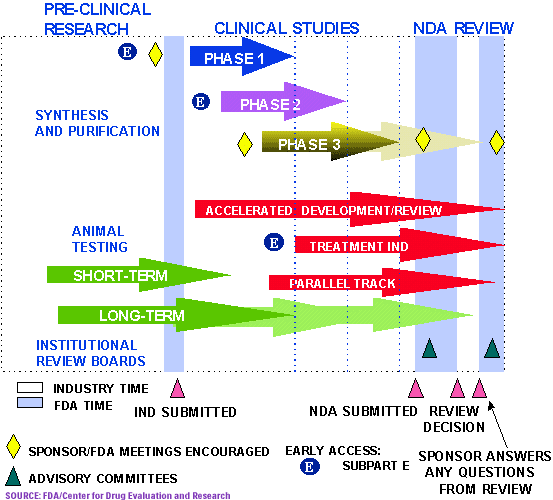
The Food and Drug Administration (FDA) plays a crucial role in safeguarding public health by ensuring the safety, efficacy, and quality of a wide range of products, including drugs, medical devices, food, and cosmetics. The FDA approval process, a rigorous and multifaceted endeavor, is a critical step in bringing new products to market. This process ensures that products meet stringent standards for safety and effectiveness before they reach consumers.
Understanding the FDA Approval Process
The FDA approval process, often referred to as product authorization, involves a comprehensive evaluation of a product’s safety, efficacy, and quality. This evaluation is conducted through a series of steps, including:
1. Pre-Clinical Testing: This initial stage involves laboratory and animal studies to assess a product’s potential safety and effectiveness. Data from pre-clinical testing is crucial for informing the design of clinical trials.
2. Clinical Trials: Clinical trials are the cornerstone of the FDA approval process. These trials involve human participants and are designed to evaluate a product’s safety and effectiveness in a controlled environment. Clinical trials are conducted in phases, with each phase building upon the previous one:
- Phase 1: Small-scale trials designed to assess a product’s safety and determine optimal dosage.
- Phase 2: Larger trials focusing on the product’s effectiveness and safety in a specific population.
- Phase 3: Large-scale trials comparing the product’s effectiveness and safety to existing treatments or a placebo.
3. Regulatory Review: Once clinical trials are completed, the manufacturer submits a comprehensive application to the FDA. The agency then reviews the application, assessing the data from pre-clinical testing and clinical trials to determine if the product meets the established safety and efficacy standards.
4. Approval or Rejection: The FDA can approve the product, approve it with specific conditions, or reject the application. The decision is based on a thorough evaluation of the submitted data and the product’s potential risks and benefits.
5. Post-Market Surveillance: Even after a product is approved, the FDA continues to monitor its safety and effectiveness through post-market surveillance. This involves collecting data from various sources, including healthcare providers, patients, and manufacturers, to identify any potential issues or adverse effects.
The Importance of FDA Approval
The FDA approval process serves several critical functions:
- Protecting Public Health: By ensuring the safety and efficacy of products, the FDA safeguards consumers from potentially harmful products.
- Promoting Innovation: The rigorous approval process incentivizes manufacturers to develop innovative and safe products, fostering advancements in medicine and healthcare.
- Building Consumer Confidence: FDA approval provides consumers with assurance that products meet specific quality and safety standards, fostering trust in the marketplace.
Benefits of FDA-Approved Products
Consumers can benefit from the FDA approval process in several ways:
- Increased Safety: FDA-approved products have undergone rigorous testing and evaluation, minimizing the risk of potential harm.
- Improved Efficacy: The approval process ensures that products meet specific standards for effectiveness, providing consumers with confidence that they will deliver the intended results.
- Enhanced Quality: FDA approval mandates adherence to stringent quality standards, ensuring consistency and reliability in product performance.
FAQs About FDA Approval
Q: What are the different types of FDA approvals?
A: The FDA grants different types of approvals, including:
- New Drug Application (NDA): For new drugs intended for human use.
- Biologics License Application (BLA): For biological products like vaccines and antibodies.
- Premarket Approval (PMA): For high-risk medical devices.
- 510(k) Premarket Notification: For lower-risk medical devices.
Q: How long does the FDA approval process take?
A: The duration of the FDA approval process varies depending on the product type, complexity, and the completeness of the submitted data. It can range from a few months to several years.
Q: Can the FDA revoke approval for a product?
A: Yes, the FDA can revoke approval for a product if it is found to be unsafe or ineffective or if the manufacturer fails to comply with regulatory requirements.
Q: What are some examples of products approved by the FDA?
A: The FDA approves a vast array of products, including:
- Drugs: Antibiotics, pain relievers, chemotherapy drugs, antidepressants.
- Medical Devices: Pacemakers, artificial hips, stents, diagnostic equipment.
- Food: Processed foods, dietary supplements, food additives.
- Cosmetics: Sunscreen, makeup, skincare products.
Tips for Consumers
- Look for the FDA approval mark: The FDA approval mark is a symbol of assurance that a product has met the agency’s safety and efficacy standards.
- Consult with healthcare professionals: Healthcare professionals can provide valuable information about FDA-approved products and their suitability for individual needs.
- Stay informed about product recalls: The FDA regularly issues product recalls for products that have been found to be unsafe or defective. Consumers should stay informed about these recalls to ensure they are using safe products.
Conclusion
The FDA approval process is a vital safeguard for public health, ensuring that products meet rigorous standards for safety, efficacy, and quality. By understanding the process and its importance, consumers can make informed decisions about the products they use, promoting their well-being and contributing to a safer and healthier society.







Closure
Thus, we hope this article has provided valuable insights into The FDA Approval Process: A Gateway to Safety and Efficacy. We hope you find this article informative and beneficial. See you in our next article!
