The FDA Approval Process: Ensuring Safety and Efficacy of Medical Devices and Drugs
Related Articles: The FDA Approval Process: Ensuring Safety and Efficacy of Medical Devices and Drugs
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to The FDA Approval Process: Ensuring Safety and Efficacy of Medical Devices and Drugs. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The FDA Approval Process: Ensuring Safety and Efficacy of Medical Devices and Drugs
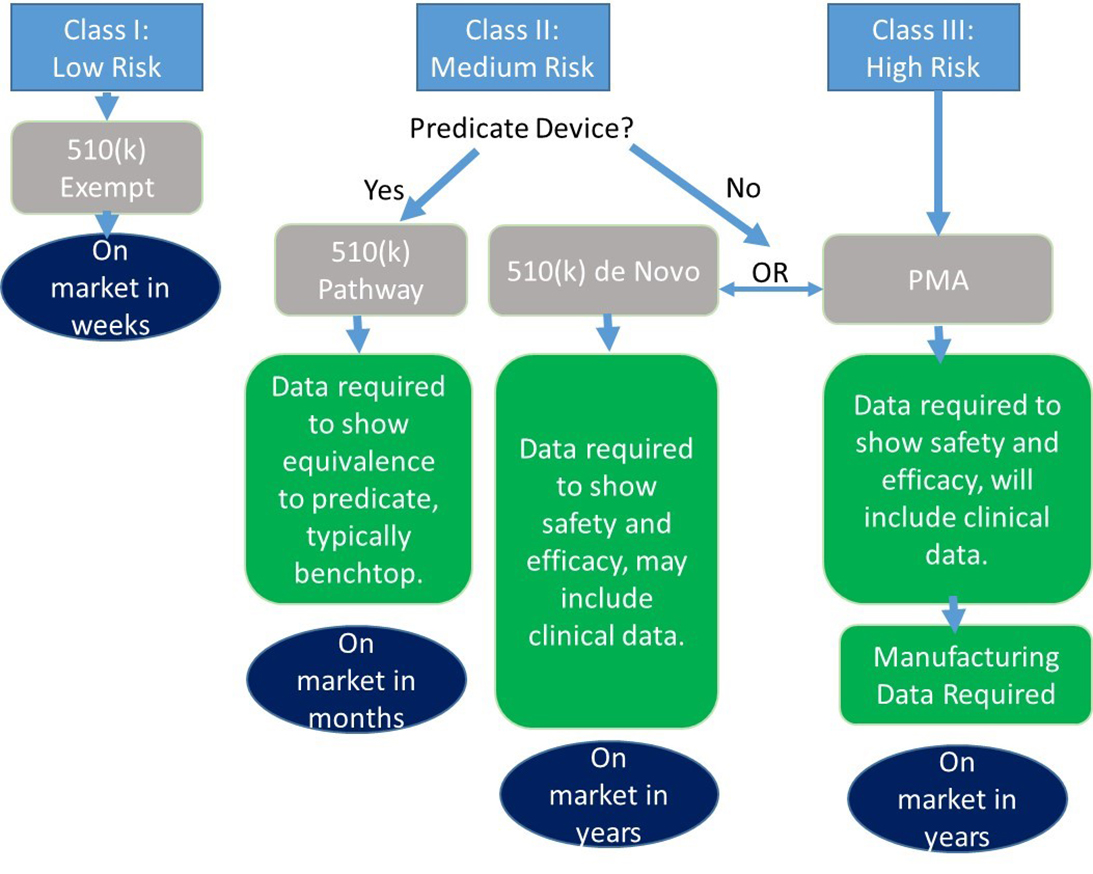
The Food and Drug Administration (FDA) plays a crucial role in safeguarding public health by ensuring the safety and efficacy of medical devices and drugs. This rigorous process involves a multifaceted evaluation of the product’s intended use, potential risks, and benefits. It is a critical step in bringing new medical innovations to market and ensuring that patients receive safe and effective treatments.
Understanding the FDA Approval Process
The FDA approval process is a complex and comprehensive system designed to evaluate the safety, effectiveness, and quality of medical products. It involves a series of stages, each with specific requirements and criteria.
1. Pre-Market Activities:
- Preclinical Testing: This stage involves laboratory and animal studies to assess the safety and efficacy of the product. Data from these studies are compiled into a pre-Investigational New Drug (IND) or Investigational Device Exemption (IDE) application, which is submitted to the FDA for review.
- Clinical Trials: Once the FDA approves the pre-IND or IDE application, the product can be tested in humans. Clinical trials are conducted in phases, starting with small groups of healthy volunteers and progressively expanding to larger groups of patients with the targeted condition.
- Data Collection and Analysis: Throughout the clinical trial phases, researchers meticulously collect data on the product’s effectiveness, side effects, and overall safety. This data is analyzed and presented in a comprehensive report.
2. Submission and Review:
- New Drug Application (NDA) or Premarket Approval (PMA) Application: Once clinical trials are complete, the manufacturer submits a detailed application to the FDA. The NDA or PMA application includes comprehensive data on the product’s safety, efficacy, manufacturing processes, and labeling.
- FDA Review: The FDA rigorously reviews the submitted data, often involving a team of scientists, medical experts, and regulatory specialists. They assess the product’s safety, effectiveness, and quality, considering all potential risks and benefits.
- Advisory Committee Meetings: In some cases, the FDA may convene an advisory committee of independent experts to provide recommendations on the product’s approval. These meetings are open to the public and offer transparency in the decision-making process.
3. Post-Market Surveillance:
- Continued Monitoring: Even after a product is approved, the FDA continues to monitor its safety and effectiveness. This includes analyzing data from post-market surveillance programs, reports from healthcare providers, and patient feedback.
- Adverse Event Reporting: The FDA encourages healthcare providers and patients to report any adverse events or side effects associated with the product. This information helps identify potential safety concerns and adjust labeling or recommendations as necessary.
Benefits of FDA Approval
- Safety: FDA approval ensures that medical products meet rigorous safety standards, minimizing the risk of harm to patients.
- Efficacy: The approval process verifies that the product works as intended, providing patients with effective treatments.
- Quality: The FDA mandates quality control measures throughout the manufacturing process, ensuring that products meet high standards of purity and consistency.
- Transparency: The FDA approval process is transparent, allowing public access to information about approved products and their potential risks and benefits.
- Consumer Confidence: FDA approval provides consumers with confidence in the safety and effectiveness of medical products, promoting informed decision-making.
FAQs about FDA Approval
Q: How long does the FDA approval process take?
A: The duration of the FDA approval process varies significantly depending on the complexity of the product and the type of application. It can range from a few months to several years.
Q: What are the criteria for FDA approval?
A: The FDA evaluates the product’s safety, efficacy, and quality, considering factors such as:
- Intended Use: The product must be safe and effective for its intended use.
- Risk-Benefit Profile: The potential benefits of the product must outweigh the potential risks.
- Manufacturing Process: The manufacturing process must be robust and ensure the product’s quality.
- Labeling: The product’s labeling must be clear, accurate, and provide essential information for patients and healthcare providers.
Q: What happens if a product is not approved by the FDA?
A: If the FDA determines that a product does not meet the required safety, efficacy, or quality standards, it will not be approved. The manufacturer may be required to conduct further studies or modify the product before resubmitting an application.
Q: Can FDA-approved products be recalled?
A: Yes, even FDA-approved products can be recalled if safety concerns arise after market release. The FDA can issue recalls for products that pose a significant risk to public health.
Tips for Understanding FDA-Approved Products
- Read the Product Labeling: Carefully review the product’s labeling, including the package insert, for information on its intended use, potential side effects, and warnings.
- Talk to Your Healthcare Provider: Discuss any concerns you have about the product with your healthcare provider. They can provide personalized advice and answer your questions.
- Report Adverse Events: If you experience any adverse events or side effects after using a product, report them to the FDA through its MedWatch program.
- Stay Informed: Stay updated on FDA announcements and safety information regarding medical products.
Conclusion
The FDA approval process is a vital safeguard for public health, ensuring the safety and efficacy of medical devices and drugs. By rigorously evaluating products and monitoring their performance after market release, the FDA helps to protect patients and promote the development of innovative and effective treatments. Understanding the FDA approval process and its importance empowers individuals to make informed decisions about their healthcare and contribute to a safer and healthier society.


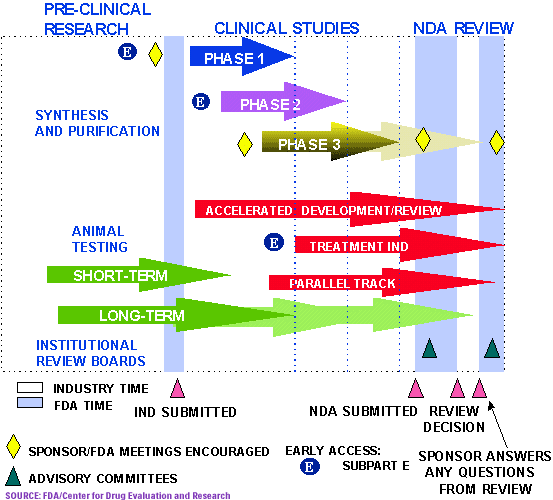

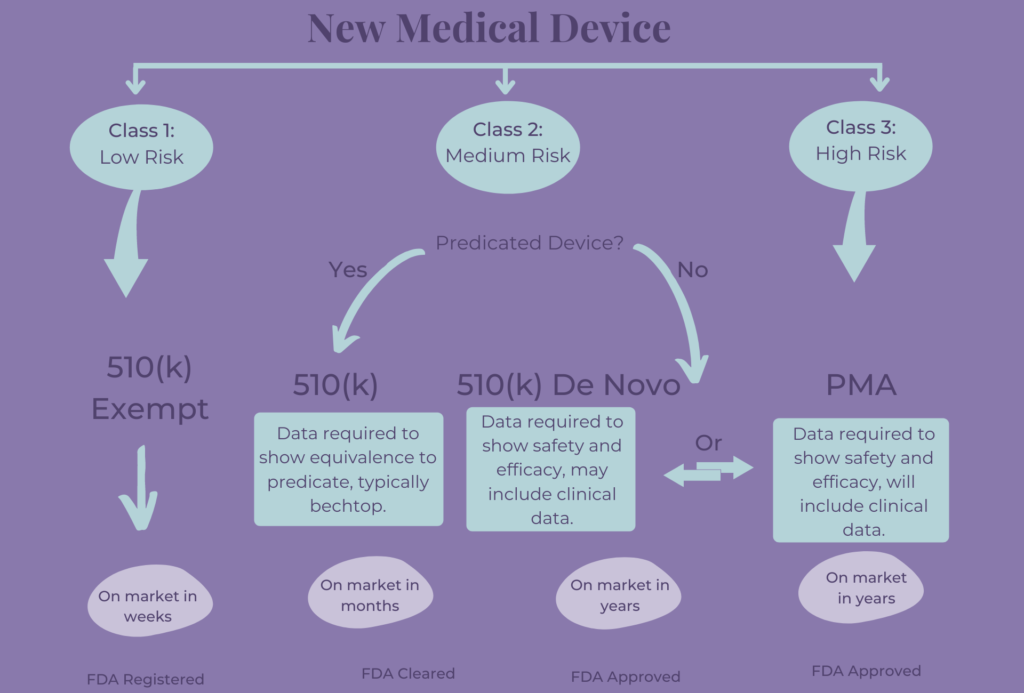


Closure
Thus, we hope this article has provided valuable insights into The FDA Approval Process: Ensuring Safety and Efficacy of Medical Devices and Drugs. We appreciate your attention to our article. See you in our next article!